New paper describes on-surface synthesis and characterization of nitrogen-substituted undecacenes
by Carey Sargent, EPFL, NCCR MARVEL
Acenes have attracted a great deal of interest because they feature remarkable electronic properties such as spin-polarization and magnetism in the larger members of the family. With increasing length, the energy gap between the acene’s highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) reduces rapidly—anthracene and tetracene, acenes with three and four linearly fused benzene rings, respectively, already possess semiconducting properties.
The small electronic gap together with high electron mobility makes the materials attractive for use in applications such as organic field-effect transistors (OFETs), organic light-emitting diodes (OLEDs), organic photovoltaic (OPV) and spintronic devices. The materials could also be used to significantly increase the photoconversion efficiency of organic photovoltaic devices. Beyond these potential applications, acenes, especially higher acenes, that is, those with six or more linearly fused benzene rings, provide an opportunity for exploring π-electron correlation and magnetism effects: they feature a unique topology of the π-bond system, that is formed by the overlap of the pz orbitals of carbon atoms.
Meanwhile, heteroatom substitution, in which carbon atoms are replaced with atoms such as boron, phosphorus, oxygen, sulfur or nitrogen in the acene, is an effective way of tuning electronic, magnetic, and physico-chemical properties. In acenes, replacing CH groups with such heteroatoms produces oligoheteroacenes and the properties of these materials depend on the type, number, and position of the heteroatoms.
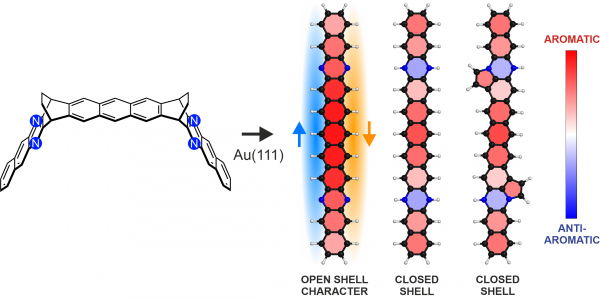
Figure 1: From a specifically designed novel diethano-bridged precursor molecule including four nitrogen atoms, on-surface chemistry was used to synthesized three different nitrogen-substituted undecacenes: (left to right) tetraazaundecacene, hydrogenated tetraazaundecacene and its analog with edge-fused five-membered rings. Tetraazaundecacene was found to exhibit considerable open-shell character, with unpaired electrons along the two zigzag edges, indicated by the blue and orange arrows. Rings are colored based on local aromaticity, calculated with the NICSπzz(1) method, showing overall aromaticity and local antiaromaticity induced by the hydrogenated nitrogen atoms.
Boron and nitrogen substitution has, for example, been shown to offer precise control over the radical character and chemical stability of acenes. If all the heteroatoms are nitrogen, the compounds are called azaacenes (or N-heteroacenes). Such compounds inherit the remarkable properties of acenes because the nitrogen atom leaves the topology of the π-system equivalent with respect to the substituted CH group. Electronegative nitrogen atoms allow for finely tunable frontier molecular orbital energy alignment and azaacenes have been shown to exhibit n-type semiconducting behavior and have been used in thin-film transistors, OLEDs and in OPV devices.
Synthesizing and characterizing larger acenes has been a challenge for solution chemistry though because they feature low solubility and high reactivity. There has been some success with synthesizing higher acenes, but such compounds may need to be stabilized in a matrix to keep them from decomposing after a few hours or made with the help of various bulky protecting groups.

On-surface chemistry has emerged as an alternative method that allows for the synthesis of new organic materials by one- or two-dimensional spatial confinement on single-crystal substrates under ultrahigh vacuum (UHV) conditions. The molecular structure and electronic properties of the targeted nanomaterials can be identified via advanced scanning probe techniques, such as scanning tunneling microscopy/spectroscopy (STM/STS) and non-contact atomic force microscopy (nc-AFM). In the last decade, the on-surface synthesis of graphene nanoribbons (GNRs), the discovery of novel chemical reactions, and the synthesis of carbon-based nanographenes with intriguing electronic properties have contributed significantly towards the development of this method.
More recently, researchers have turned to the synthesis and characterization of higher acenes on surfaces: the largest so far have been those with 10, 11 or 12 benzene rings. Despite these successes and the existence of on-surface approaches to synthesizing heteroatom-substituted graphene-derived systems, scientists haven’t investigated higher heteroacenes, however. Likewise, there is still no clear scientific consensus on the nature of the ground state of higher acenes: many computational studies have found it to be open-shell polyradical, though a recent quantum Monte Carlo study predicted it to be a closed-shell ground state.

In the paper On-surface synthesis and characterization of nitrogen-substituted undecacenes, recently published in Nature Communications, a team of researchers led by Dr. Carlo Pignedoli, Deputy Group Leader Atomistic Simulations at Empa, and Kristjan Eimre, PhD student in Empa’s nanotech@surfaces Laboratory, demonstrated the on-surface formation of hydrogenated tetraazaundecacene, its analog with two edge-fused five-membered rings, and the 6,12,19,25-tetraazaundecacene via thermal annealing and STM tip manipulation of a novel-design precursor molecule on a coinage metal surface under UHV conditions. They also showed that a similarly designed precursor can be used to synthesize the pristine undecacene.
They were able to assign an experimental ground state with considerable open-shell character for the synthesized tetraazaundecacene on Au(111), while the other two compounds were found to be closed-shell. Calculations based on DFT demonstrate that the tetraazaundecacene retained the remarkable electronic properties of the pristine undecacene except for a downshift in the orbital energies introduced by the electronegative nitrogen atoms. Additionally, calculations show that the hydrogenated analogs exhibit local antiaromaticity induced by the nitrogen atoms.
The procedure offers a new perspective for investigating complex electronic correlation effects in acenes by chemical substitution, opening a new path toward precisely tailored building blocks of organic electronics and spintronics, the researchers said.
Reference:
Eimre, K., Urgel, J.I., Hayashi, H. et al. On-surface synthesis and characterization of nitrogen-substituted undecacenes. Nat Commun 13, 511 (2022).
Low-volume newsletters, targeted to the scientific and industrial communities.
Subscribe to our newsletter