Computer simulations unveil phase transitions in supercapacitors materials
By Nicola Nosengo/NCCR MARVEL
Electrification is a fundamental step towards reducing carbon emissions, and its success relies on improving the technologies we use to generate, store and distribute electricity. Among these technologies are supercapacitors, devices that can store electrical charge and discharge it very quickly and that have applications from the automotive industry to renewable energy storage. Like batteries, supercapacitors have two electrodes and an electrolyte in between. Unlike batteries, they do not rely on chemical reactions to release energy, but simply on the interaction between free charges in the electrolyte and the voltage applied to the electrodes.
Finding the best electrolytes is key to build supercapacitors with higher capacitance (that is, the ability to store electrical charge), longer operational life and lower cost. In a study published in Proceedings of the National Academy of Sciences (PNAS), a research team led by CECAM deputy director at EPFL and MARVEL member Sara Bonella, has shown that the electrolyte behavior in proximity of the electrode may undergo complex phase transitions that drastically differ from the bulk liquid behavior– a key step to predict the performance of supercapacitors.
The research team behind this work is composed, in addition to Bonella, by Federica Angiolari from CECAM and EPFL, Alessandro Coretti from the University of Vienna and Mathieu Salanne from Sorbonne University. The team’s initial goal was to use state-of-the-art materials simulation methods to study the phase behavior of adsorbed electrolytes when different electric potentials are applied on the two electrodes. The authors used molecular dynamics – a computer simulation method that uses Newton’s laws to model the movements of atoms and molecules– to an ideal supercapacitor with aluminum electrodes and a liquid electrolyte, a molten salt of lithium and chloride. It’s a supercapacitor that cannot exist in the real world, because aluminum would melt at the temperature required to keep the electrolyte in a liquid state. But it shares most properties of real systems while being easier to simulate.
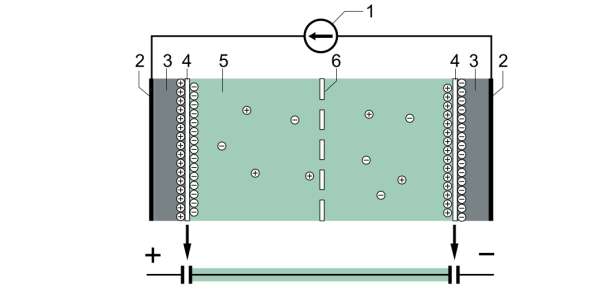
Typical construction of a supercapacitor: (1) power source, (2) collector, (3) polarized electrode, (4) Helmholtz double layer, (5) electrolyte having positive and negative ions, (6) separator. Source: Tosaka/Wikimedia. CC BY 3.0
The results of the calculations surprised the scientists. “In a sense we started out to reach India, but we ended up discovering America”, jokes Bonella. The team found out that the structures formed by the electrolyte at the interface undergo a two-stage phase transition, unlike what happens in liquids. In the latter, the arrangement of the atoms changes abruptly, going from a disordered arrangement towards an ordered one – similarly to what happens when water freezes, and the disordered arrangement of water molecules is replaced by the organized crystal structure. In the present study, the transition occurs in two stages, and an intermediate phase is observed in which they adopt orientational ordering only.
Phase transitions in an electrolyte are important, because they can dramatically change the capacitance of the device. “They had been observed in two-dimensional systems, but for ionic materials no one had seen them”, says Bonella. “There were some theoretical results on very small systems, but not very accurate ones. The fact that we found phase transitions in a much more realistic simulation makes us suspect that they are a more generalized phenomenon than previously thought”.
The key innovation of the study was to use a “classical” simulation, involving no quantum calculation of electronic structure involved, that mimics the metallic nature of the electrodes. “Even though it does not explicitly represent electrons, it can show how charge is redistributed across the electrodes”, says Federica Angiolari, the postdoctoral fellow responsible for this work thanks to funding from the MARVEL Agility program. As for molecular dynamics, the team used a variant called Mass Zero molecular dynamics (MaZe), developed in Bonella’s lab, that allows to study large physical systems rather than the simple ones that are often chosen to lower the computational cost of simulations. “When you are forced to study small systems with few atoms, you can get a lot of artifacts” says Angiolari. “The use of MaZe and a classical model allowed us to increase the size significantly and get more accurate results”.
In the future, the same method can be used to study how the properties of the electrolyte change the behavior the interface. The team plans to study more complicated electrolytes, for example made of three chemical elements instead of two, and see what happens. “Will we still find a phase transition, but at different voltages and temperatures, or will we see something radically different?” asks Bonella.
Overall, she says, the study provides the community with a protocol to predict with unprecedented detail the interaction between electrolytes and electrodes in a supercapacitor, which could guide the work of experimentalists towards more efficient devices.
Reference
F. Angiolari,A. Coretti,M. Salanne, & S. Bonella, Electrically driven first-order phase transition of a 2D ionic crystal at the electrode/electrolyte interface, Proc. Natl. Acad. Sci. U.S.A. 122 (46) e2520026122 (2025) doi: https://doi.org/10.1073/pnas.2520026122
Low-volume newsletters, targeted to the scientific and industrial communities.
Subscribe to our newsletter