Computationally designed material shows improved carbon capture in wet flue gases
By Carey Sargent, NCCR MARVEL, EPFL
Carbon capture and storage is a technology that has gained a lot of attention—it is one of the few viable technologies for mitigating CO2 emissions. A corresponding effort has been placed on developing solid adsorbents that can efficiently capture CO2 from flue gases emitted from, for example, factories and powerplants.
Metal–organic frameworks (MOFs), a combination of organic ligands and metal-ion nodes, have attracted a lot of interest in this application because they can in principle be assembled to form many structurally and chemically distinct nanoporous materials.
Of those that have been optimized for the separation of CO2 from nitrogen, however, few perform well with realistic flue gases that contain water. This is because water competes with CO2 for the same adsorption sites in these materials and causes them to lose their selectivity. Flue gases can be dried but this makes the capture process prohibitively expensive.
In the paper Data-driven design of metal–organic frameworks for wet flue gas CO2 capture, the researchers developed a systematic strategy for the design and preparation of custom-made MOFs that can capture carbon from wet flue gases. Inspired by the rational design of drug molecules, an approach that involves mining databases for organic molecules that fit into the binding pocket of a given protein well, their design methodology involved generating a library of 325,000 hypothetical MOFs and screening then each for its CO2/N2 selectivity and CO2 working capacity.
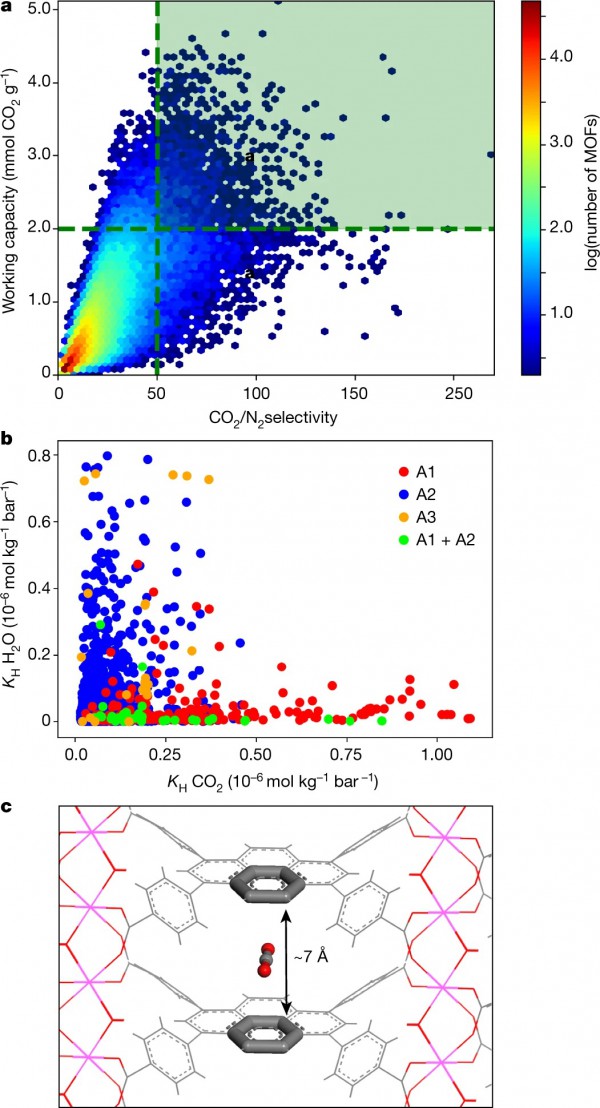
a, Results from the screening of 325,000 hypothetical MOFs under conditions that mimic post-combustion capture. The materials in the green box were selected for more refined screening and adsorbaphore identification; the colour-coding represents the number of MOFs according to the colour bar on the right. b, The H2O affinity of the top-performing materials, as characterized by a plot of the Henry coefficients (KH) for H2O against those of CO2. The colours represent the three different adsorbaphores found in the top-performing structures: A1, parallel aromatic rings; A2, metal–oxygen bridges; A3, open metal sites. c, The adsorbaphore containing parallel aromatic rings (A1), which was discovered using the feature-recognition algorithm.
They identified different classes of strong CO2-binding sites—which they refer to as term ‘adsorbaphores’—that allow MOFs to retain their CO2/N2 selectivity even in wet flue gases. They then synthesized two water-stable MOFs containing the most hydrophobic adsorbaphore. The carbon-capture performance was not affected by water and outperformed that of some commercial materials. Identifying the optimal separation material will require testing the performance of these MOFs in an industrial setting and considering the entire capture process, including the targeted end use of the harvested CO2.
Reference:
Peter G. Boyd, Arunraj Chidambaram, Enrique García-Díez, Christopher P. Ireland, Thomas D. Daff, Richard Bounds, Andrzej Gładysiak, Pascal Schouwink, Seyed Mohamad Moosavi, M. Mercedes Maroto-Valer, Jeffrey A. Reimer, Jorge A. R. Navarro, Tom K. Woo, Susana Garcia, Kyriakos C. Stylianou, Berend Smit. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture. Nature 11 December 2019. DOI: 10.1038/s41586-019-1798-7
Low-volume newsletters, targeted to the scientific and industrial communities.
Subscribe to our newsletter